TOPIC INFO (UGC NET)
TOPIC INFO – UGC NET (Geography)
SUB-TOPIC INFO – Geomorphology (UNIT 1)
CONTENT TYPE – Short Notes
What’s Inside the Chapter? (After Subscription)
1. Denudational Processes
1.1. Concept
2. Chemical Weathering
2.1. What exactly is Chemical Weathering?
2.2. Process of Chemical Weathering
2.3. Natural Dissolution
2.4. Solution Weathering
2.5. Carbonation Weathering
2.6. Hydration
2.7. Hydrolysis
2.8. Oxidation and Reduction
3. Physical Weathering
3.1. What is Physical Weathering?
3.2. Various Mechanisms of Physical Weathering
3.3. Types of Physical Weathering
3.4. Significance of Physical Weathering
4. Biological Weathering
4.1. What is Biological Weathering?
4.2. Types of Biological Weathering
4.3. Classification
5. Significance of Weathering
6. Factors Affecting Weathering Processes
6.1. Climate
6.2. Rock Type
6.3. Slope Variation
6.4. Vegetation
7. Mass Movements
7.1. Slow Movements
7.2. Rapid Movements
7.3. Erosion (Transportation) and Deposition
Note: The First Topic of Unit 1 is Free.
Access This Topic With Any Subscription Below:
- UGC NET Geography
- UGC NET Geography + Book Notes
Denudation and Weathering
UGC NET GEOGRAPHY
Geomorphology (UNIT 1)
Denudational Processes
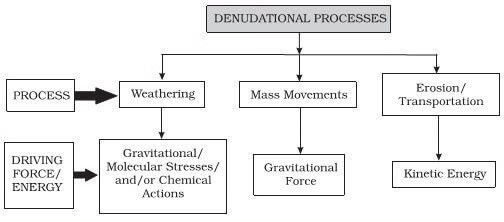
Denudation process is a broad term that encompasses all exogenic processes (weathering and erosion). The term ‘denude’ refers to the process of removing or uncovering something. Denudation processes are influenced by physical (folds, faults, orientation, inclination of beds, presence or absence of joints, bedding planes, hardness or softness of constituent minerals, permeability) and chemical (chemical susceptibility of mineral constituents to corrosion) properties of the rocks.
Concept
- Denudational processes refer to the combined actions that result in the wearing down or stripping away of the Earth’s surface layers.
- These processes are critical in shaping landscapes and are a subset of the broader category of exogenic or external geomorphic processes.
- Denudation encompasses several sequential processes:
- Weathering: This is the initial breakdown of rocks at or near the Earth’s surface due to various physical, chemical, and biological agents. Weathering does not involve the transportation of eroded materials.
- Erosion: This involves the removal and transportation of weathered material by various agents like water, wind, ice, and gravity. Different types of erosion lead to diverse landforms and landscapes.
- Transportation: The movement of eroded materials by agents like rivers, winds, glaciers, and waves. Different agents have specific modes of transport, leading to varied landforms.
- Deposition: This is the process by which transported materials settle or get laid down in new locations. Over time, the accumulation of these deposited materials can give rise to landforms such as deltas, alluvial fans, beaches, and moraines.
Chemical Weathering
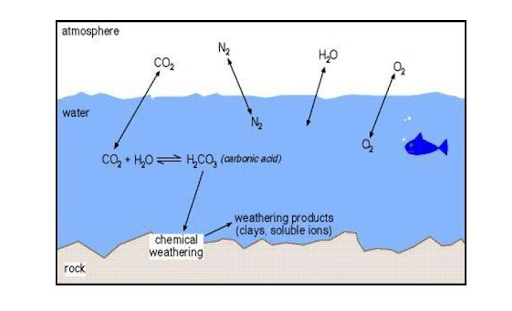
Chemical weathering is the process of chemical decomposition of rocks and soil. Chemical weathering involves the rock interacting with chemicals to alter the rock’s composition. The chemical weathering processes such as dissolution, solution, carbonation, hydration, oxidation, and reduction operate on rocks to break down, dissolve, or reduce them to a fine state.

What exactly is Chemical Weathering?
- Chemical Weathering refers to the breakdown and decomposition of rocks and minerals at the Earth’s surface as a result of chemical reactions with the atmosphere, moisture, and organic materials.
- Unlike physical or mechanical weathering, which primarily changes the size and shape of rocks without altering their composition, chemical weathering changes the chemical composition of rocks.
- All chemical reactions are sped up by acids created by microbial and plant-root metabolism, water, air (oxygen and carbon dioxide), and heat.
- Through chemical reactions involving oxygen, surface and/or soil water, and other acids, a range of weathering processes known as solution, carbonation, hydration, oxidation, and reduction operate on rocks to breakdown, dissolve, or reduce them to a fine clastic state.
- To accelerate all chemical processes, water, air (oxygen and carbon dioxide), and heat must be present.
- In addition to the carbon dioxide in the air, the breakdown of plants and animals increases the amount of carbon dioxide underground.
- These chemical reactions on various minerals are quite comparable to laboratory chemical reactions.
Process of Chemical Weathering
- After forming inside the crust of Earth for millions of years, they reach the surface due to denudation and other such measures.
- Once they are on the surface, they come in contact with different ecological factors like water, air, etc.
- These factors make changes in the rocks. This can be either physical weathering or chemical weathering.
- Chemical weathering changes the structure of the rocks due to some form of chemical reaction on them.
- Agents like water, or acids (from acid rain), can react with specific elements present in the rock which reduces its strength leading to its breaking.
- After breaking, these are either carried away by agents like wind, air, etc., or get transformed into some other element, or are used up by the surroundings.
- They bring changes in the molecular structure of the rocks.
Classification of Chemical Weathering:
Natural Dissolution
- Dissolution is the process of a gaseous, liquid, or solid solute dissolving in a solvent to form a solution.
- Due to their natural solubility (such as nitrates, sulfates, and potassium) and oxidation potential, iron-rich minerals will weather naturally through dissolution (rains).
- These minerals are easily leached out and accumulate in dry areas without leaving residue behind.

Natural Dissolution
Solution Weathering
- When the solvent is an acidic solution rather than plain water, solution weathering happens.
- A solution is a liquid mixture in which the solute (small component) is evenly distributed inside the large component (the solvent).
- Any solution with a higher concentration of hydrogen ions than water is considered acidic; solutions with a lower concentration of hydrogen ions than water are either basic or alkaline.

Solution Weathering
Carbonation Weathering
- Carbonation is the process of converting carbon dioxide into carbonates, bicarbonates, and carbonic acid.
- The process of atmospheric carbon dioxide-causing solution weathering is known as carbonation weathering.
- Rain dissolves small amounts of carbon dioxide from the air, generating a weak acid capable of dissolving minerals such as limestone (calcium carbonate).
- Caves arise when carbonic acid-containing subsurface water passes through limestone blocks, dissolves the limestone, and leaves empty pockets (caves) behind (Karst topography).
- Since cooler water stores more dissolved carbon dioxide gas, the carbonation process accelerates as the temperature drops.

Carbonation Weathering
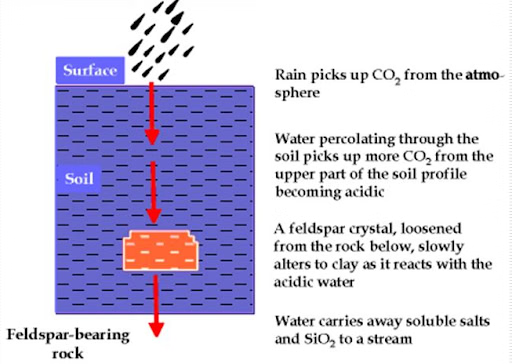
Hydration
- Hydration is the chemical addition of water to atoms and molecules of a mineral, involving the hard attachment of H+ and OH- ions to the atoms and molecules.
- The increased volume created by rock minerals absorbing water produces physical tensions within the rock.
- Iron oxides, for example, are transformed into iron hydroxides, which have a higher volume.
- Since hydration is reversible, the rocks become fatigued and may disintegrate if the procedure is repeated continuously.
Hydration Weathering
Hydrolysis
- A water molecule is consumed in biological hydrolysis to impact the separation of a bigger molecule into component pieces.
- Pure water combines with silicate or carbonate minerals in biological hydrolysis, completely dissolving the original material (dissolution weathering).
- Biological hydrolysis is an essential response in regulating CO2 levels in the atmosphere and it can have an impact on climate.
Hydrolysis
Oxidation and Reduction
- In the case of weathering, oxidation means the combination of minerals and oxygen to form oxides (rust in the case of iron) or hydroxides.
- Due to the presence of iron oxide, red soil looks red.
- Oxidation occurs in places with easy access to air and water.
- The most commonly involved minerals in this process are iron, manganese, and sulfur.
- Reduction occurs when oxidized minerals are placed in a non-oxygenated environment. Such conditions usually exist below the water table in areas of stagnant water or moist soil.
- The red color of iron turns greenish-gray or bluish-gray when reduced.
